Ethics and Pharmaceutical Industry – Product Development and Production – Asrar Qureshi’s Blog Post #795
Ethics and Pharmaceutical Industry – Product Development and Production – Asrar Qureshi’s Blog Post #795
Dear Colleagues! This is Asrar Qureshi’s Blog Post #795 for Pharma Veterans. Pharma Veterans welcome sharing of knowledge and wisdom by Veterans for the benefit of Community at large. Pharma Veterans Blog is published by Asrar Qureshi on WordPress, the top blog site. Please email to asrar@asrarqureshi.com for publishing your contributions here.
 |
| Google Images |
 |
| Google Images |
 |
| Google Images |
Production
Production comes next to product development. Formulations are developed and tested for optimization of various excipient to ensure maximum bioavailability. Bioavailability means how much of the given drug gets into the blood after absorption, from where it is distributed to various tissues. It is easy to understand that injections have 100% bioavailability, because these are directly (intravenous) or indirectly (intramuscular) put into the blood. However, for drugs taken orally, the absorption and bioavailability vary greatly, maybe from 5% to 99%. Research companies work on each new product formulation in a different way; generic companies do not do so and rely on certain standard formulations for every product.
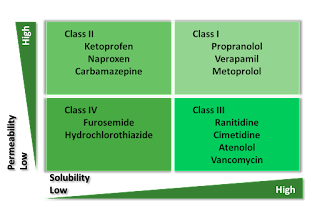
More recently, BCS – Biopharmaceutical Classification System has come under discussion. BCS is a prognostic tool for assessing the effect of formulation on oral drugs given to humans. BCS Class I – IV depicts solubility and permeability (into the intestinal tissue to reach the blood) along X-axis and Y-axis. BCS class pertains to the Active Pharmaceutical Ingredient – API.
• BCS class I High Solubility, High Permeability
• BCS class II Low Solubility, High Permeability
• BCS class III High Solubility, Low Permeability
• BCS class IV Low Solubility, Low Permeability
As may be seen, BCS class IV drugs are most difficult to formulate otherwise their efficacy will not be ensured.
Someone may ask, why do we need to formulate? Why not just take the active ingredient as such, maybe as a powder in a capsule to avoid bitter taste. The answer is, it will not be absorbed and shall pass out as such. Aspirin and Paracetamol are BCS Class I drugs and are easy to formulate. Codeine and morphine are Class II drugs; Ciprofloxacin is a Class III drug and more difficult to formulate. Furosemide and sulfamethoxazole (one ingredient in the famous Septran) are Class IV drugs. I may repeat that it is easiest to formulate Class I drugs and hardest to formulate Class IV drugs. This is relatively new knowledge though many of these drugs had been available for decades.
My readers would have understood the importance of formulation and also the need for customizing formulation for each product. Whatever happened in the past cannot be undone but the future can be made better.
Another important conclusion is that of fixing not just the formulation but also the ingredients. This is another sensitive area because change of source may affect the behavior of the drug.
I may also handle one question here which is asked repeatedly by common people. Question, “It is usually seen that drugs imported from US and Europe are more effective compared to the same drugs made in Pakistan”. The observation is not entirely wrong. However, I must category clarify that it is not because the quantity of active drug.
There are possibly three reasons which may contribute to this effect. One, the quality of active ingredient varies even within the same pharmacopeial specifications range. The APIs supplied to the US and European markets have more stringent specs as compared to those supplied to third world countries. The prices also vary accordingly. These countries are more focused on drug prices, not other factors. Two, the formulations are optimized for drugs manufactured in the developed countries, while drugs manufactured in countries like ours are more or less based on standard formulations. Three, drugs are manufactured on sophisticated, state-of-the-art equipment which ensures precision of the manufactured drug, while our plants are old with imprecise equipment. These are apparently small differences, but together these may make tangible difference. I would again emphasize that focus on price may compromise efficacy and safety of a drug. Government, who regulates the drug prices, is also responsible, not just the manufacturers.
Production has undergone many developments over the years. New guidelines, rules, have been established by the international regulatory bodies. World Health Organization has provided training to regulators in LMICs at its own cost. The overall system of regulations is being constantly strengthened. All this had had a positive impact on the quality of drugs.
Production units have also come a long way in terms of technology and automation. Pharma companies who got into business in the early years of independence, had very basic manufacturing units in which equipment was run manually. Growth of business brought more revenue and progressive businessmen invested in upgrading their units. Many units were shifted from small places in areas which had become congested with population increase, to more spacious places and/or industrial areas. Ministry of health also played a positive part in not just advising but actively supporting the local pharma industry to move upward. Some criticism is also due to the MoH for unnecessary protecting poor plants for too long and giving them license renewals when it was not entirely justified on merit.
Though I cannot put a percentage on it, but most plants have upgraded to match basic, modern requirements. Some are still resisting to change. A decade ago, most alternative medicine plants were shabby to the point of being unfit for production, but since the introduction of Health & OTC act, things are getting better. The job of regulators is one part monitoring, one part policing, and one part helping the industry to upgrade. Historically, our regulators only did policing and whatever came with it for appeasement, but younger, more trained ones are using a different approach which is welcome.
We are far from having reached where we should be at this time, but the progress is positive and better outcomes are expected.
Concluded.
Disclaimer: Most pictures in these blogs are taken from Google Images and Pexels. Credit is given where known; some do not show copyright ownership. However, if a claim is lodged at any stage, we shall either mention the ownership clearly, or remove the picture with suitable regrets.
References.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4476996/
https://www.researchgate.net/figure/List-of-drugs-classified-in-classes-1-and-3-of-BCS_tbl3_287488870



Comments
Post a Comment