New Diabetes Drug – Facts Vs Misinformation – Asrar Qureshi’s Blog Post #838
New Diabetes Drug – Facts Vs Misinformation – Asrar Qureshi’s Blog Post #838
Dear Colleagues! This is Asrar Qureshi’s Blog Post #838 for Pharma Veterans. Pharma Veterans aims to share knowledge and wisdom from Veterans for the benefit of Community at large. Pharma Veterans Blog is published by Asrar Qureshi on WordPress, the top blog site. Please email to asrar@asrarqureshi.com for publishing your contributions here.
 |
A friend of mine forwarded the following text to me yesterday. It is a very catchy message for diabetic patients as it promises a revolutionary ‘treatment’ for diabetes. If you remember, I had done a blog showing that Pakistan had become number #1 country in the incidence of diabetes with over 38% population suffering from it. Pakistanis are also averse to standard treatment of diabetes and keep looking for miracles which never happen. For us, this kind of message is not just misleading, it is killing.
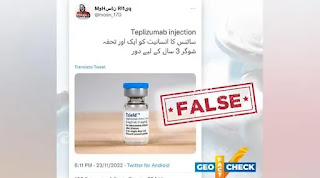
Let me tell me that this entire message is a pack of lies, misinformation, and wrong claims. The alleged drug name is TZIELD and it was approved by USFDA in November 2022.
فائزر کمپنی کی شوگر کنٹرول کرنے کی ریسرچ کردہ ویکسین نے شوگر کے مریضوں کے چہروں پر مسکراہٹیں بکھیر دیں
فائزر کمپنی نے اپنی ریسرچ ویکسین امریکہ میں متعارف کروا دی ہے جو شوگر کے مریض انسولین پر ہیں وہ ایک بار یہ ویکسین لگوائیں گے تو تین سال تک شوگر کنٹرول رہے گی اور تین ویکسین کا ڈوز لگانے کے بعد تاحیات شوگر کنٹرول رہے گی اور جو شوگر کے مریض گولیوں پر ہیں انہیں صرف ایک سنگل ڈوز لگانے سے ان کی شوگر تاحیات کنٹرول رہے گی ۔
یہ ویکسین عنقریب پاکستان میں بھی دستیاب ہو جائے گی ۔
Wrong Claim #1 – Tzield has been developed by the company PROVENTION BIO Inc. Pfizer has nothing to do with the development and approval of this drug.
Wrong Claim #2 – It is not a vaccine. It is a class of drugs called mAbs (monoclonal antibodies), which has several sub-classes, and several mAbs are already on the market for various diseases. mAbs are kind of targeted drugs.
Wrong Claim #3 – Tzield is not for patients who are fully on insulin already. It is for stage 2 Type 1 Diabetic (T1D) patients. I shall explain little later.
Wrong Claim #4 – It will not be given once to targeted patients. The dose is one injection daily intravenously for 14 days to prevent stage 2 T1D patients from going into stage 3 for a limited period of about 2 years.
Wrong Claim #5 – It will not control sugar for three years. The research has shown that it may delay the onset of T1D stage 3 for a little over 2 years.
Wrong Claim #6 – There is no lifelong sugar control. T1D patients will go into stage 3 after 2 years or so and shall have to use insulin for the rest of their lives.
Wrong Claim #7 – It is not at all meant for Type 2 diabetic patients who are taking tablets to control their diabetes.
Wrong Claim #8 – It is not expected to be available in Pakistan any time soon, mainly due to price. Provention Bio Inc. has fixed the price of one injection at US$ 13,850 (Pak Rs. 4,155,000); 14 days treatment shall cost US$194,000 (Pak Rs. 58,200,000).
Please be clear that it is not a vaccine, but it may delay a small subset of T1D stage 2 patients from going into stage 3 for up to 2.5 years. Presently, about 30,000 such patients are estimated in the US. We do not even know about the umber of such patients in Pakistan.
After debunking the false claims, let us get to the facts.
Simply speaking, Diabetes is caused by lack of insulin production in our body, or lack of its effectiveness even when it is present. Insulin is produced in the Pancreas by a certain type of cells.
Type 1 Diabetes (T1D) is an autoimmune disorder in which insulin producing cells are destroyed by the immune system of the body. Development of T1D happens in stages; stage 1 has some insulin production, stage 2 has even less, and at stage 3, patients are completely dependent on insulin injections for diabetes control. It is this narrow window when patients move from stage 2 to 3, where Tzield is claimed to help for a limited time. The patient will go into stage 3 ultimately and become insulin dependent.
Type 2 diabetes is much more common in Pakistan and elsewhere, and it is being treated with tablets effectively. It develops when the effectiveness of body’s own insulin for controlling diabetes is compromised due to several factors. The tablets given for control work in different ways, by increasing insulin production or increasing its effectiveness. Some T2D patients may have to be shifted to insulin after several years. Tzield has no role here.
For more information which may be somewhat technical, please continue to read below.
In recent years, there has been a growing interest in developing new treatments for type 1 diabetes that could slow or prevent the onset of the disease. One such treatment is Tzield (teplizumab-mzwv).
Tzield is a monoclonal antibody that is designed to bind to a protein called CD3 on the surface of T cells. T cells are a type of white blood cell that plays a role in the immune system. When T cells bind to CD3, they are activated and begin to attack foreign invaders, such as bacteria and viruses.
In people with type 1 diabetes, the immune system mistakenly attacks the body's own insulin-producing cells. Tzield works by blocking the binding of T cells to CD3. This prevents T cells from being activated and attacking the insulin-producing cells.
Tzield was approved by the U.S. Food and Drug Administration (FDA) in 2022 for the delay of the onset of stage 3 type 1 diabetes in adults and pediatric patients aged 8 years and older with stage 2 type 1 diabetes. Stage 2 type 1 diabetes is a condition in which the body has begun to attack its insulin-producing cells, but the person has not yet developed full-blown diabetes.
Tzield is given as an infusion once a day for 14 days. The treatment is most effective when given to people who are newly diagnosed with stage 2 type 1 diabetes. Studies have shown that Tzield can delay the onset of stage 3 type 1 diabetes by an average of 32.5 months.
Tzield is not a cure for type 1 diabetes, and it does not replace the need for insulin therapy. However, it is a promising new treatment that could help delay the onset of the disease and improve the quality of life for people with type 1 diabetes.
Side Effects of Tzield
Tzield may cause some side effects, including:
• Cytokine release syndrome (CRS): This is a serious allergic reaction that can occur during or after treatment with Tzield. Symptoms of CRS can include fever, chills, headache, muscle pain, nausea, vomiting, and diarrhea.
• Decrease in white blood cells: Tzield can cause a decrease in a type of white blood cell called lymphocytes. This can increase the risk of infection.
• Other side effects: Tzield may also cause other side effects, such as injection site reactions, rash, and itching.
Conclusion
Tzield is a new treatment modality for type 1 diabetes that has the potential to delay the onset of the disease. It is not a cure, but it could help improve the quality of life for people with type 1 diabetes for some time by delaying the onset of stage 3 T1D.
Concluded.
Disclaimer: Most pictures in these blogs are taken from Google Images and Pexels. Credit is given where known; some do not show copyright ownership. However, if a claim is lodged at any stage, we shall either mention the ownership clearly, or remove the picture with suitable regrets.




Comments
Post a Comment